Información
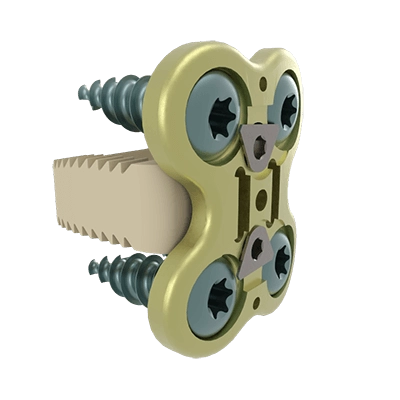
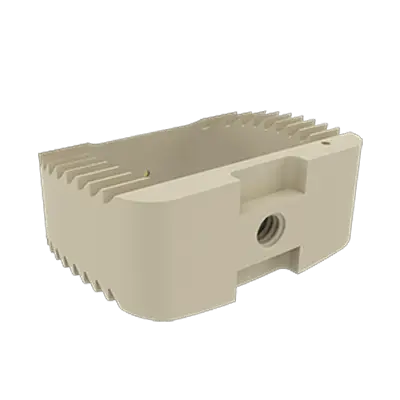
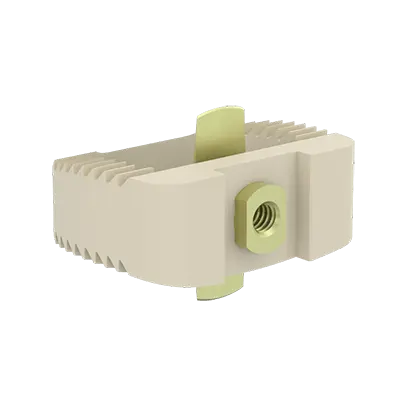
Este dispositivo es una placa cervical anterior diseñada para estabilizar el segmento vertebral durante el proceso de fusión y para fijar la caja en el espacio intervertebral.
Los implantes ACIFBOX se utilizan con instrumental específico para la preparación del lecho, la inserción y la extracción del implante.
Los implantes ACIFBOX son de un solo uso.
Materiales del implante
• Placa: Titanio (EN ISO 5832-3 ASTM F136)
• Tornillos: Titanio (EN ISO 5832-3 ASTM F136)
Materiales de los instrumentales
• Acero inoxidable, silicona y TA6V
Indicaciones
Este producto sanitario se utiliza (y sólo se utiliza) para tratar la discopatía cervical definida como radiculopatía refractaria (dolor irradiado) y/o mielopatía (debilidad) con hernia discal y/o formación de osteofitos y/o compresión de la médula espinal.
Los implantes ACIFBOX pueden utilizarse en pacientes esqueléticamente maduros (adultos) y adolescentes.