Informations
The ESP prosthetic disc is the result of 10 years of collaborative research work at the Hôpital Pitié Salpêtrière in Paris, the French Atomic Energy Commission (CEA) and the Université Paris VI, and was driven by the Oséo Innovation contest in France.
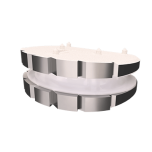
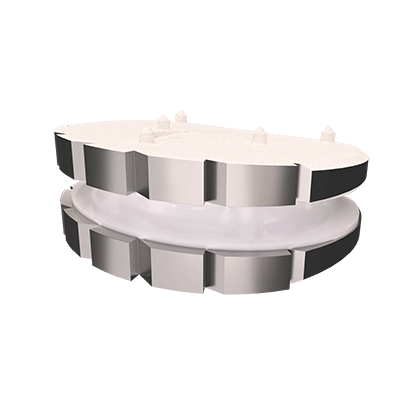
The LP-ESP® disc prosthesis is intended to replace a damaged or diseased disc between two vertebrae of the lumbar spine.
The LP-ESP® disc prosthesis is designed to reproduce the natural behavior and physiological functions of a healthy disc.
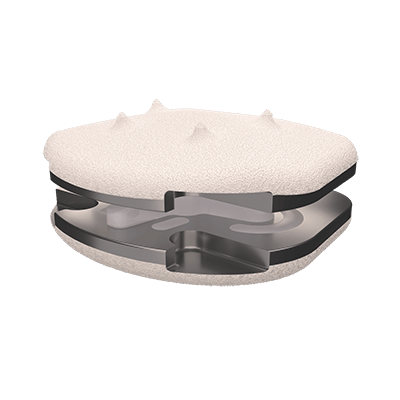
Thanks to its viscoelastic PCU core, the LP-ESP® enable mimic natural motion in all six degrees of freedom (flexion-extension, lateral bending, translation, axial rotation and shock absorption or load bear.
This flexible cushion, attached to the two metal endplates, provides the disc with its shock-absorbing function and limits the release of particles into the body and the calcification of deformable parts.
Indications
The LP-ESP® lumbar disc prosthesis is designed for the treatment of degenerative disc disease (DDD) in the lumbar column.
Disc condition is evaluated based on the convergence of data from clinical examinations and imaging.
The LP-ESP® lumbar disc prosthesis is a medical device for primary treatment.