Informations
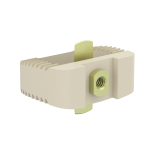
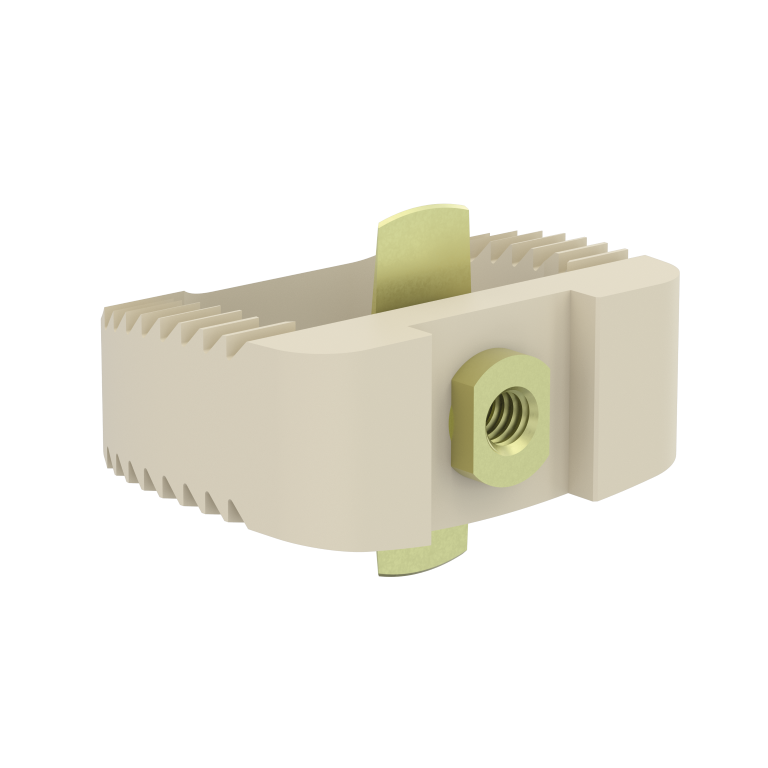
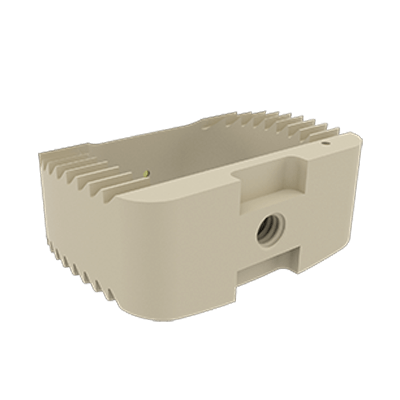
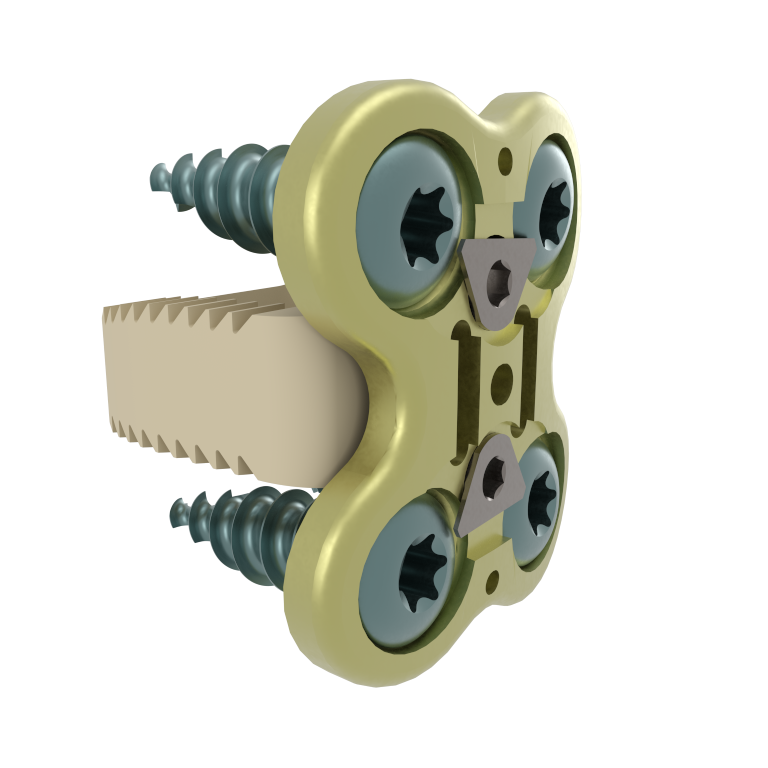
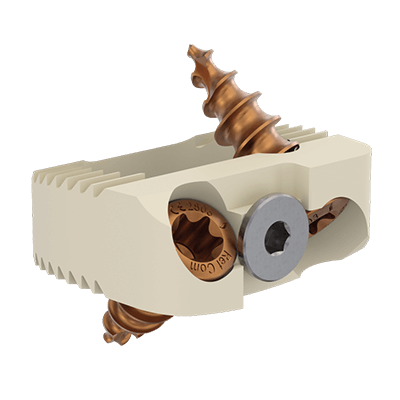
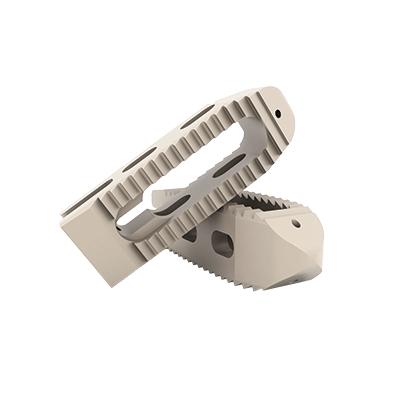
This device is an anterior intersomatic cervical cage.
It is intended to reduce the pathologies of the cervical spine by restoring the disc height as well as the physiological bending of the spine and by allowing the fusion of vertebral bodies between them.
ACIFBOX implants are used with dedicated instruments for site preparation, implant insertion and removal.
ACIFBOX implants are for single use only.
Implant material
• PEEK-OPTIMA® (ASTM F2026) + Gold (ASTM B562) + Titanium (EN ISO 5832-3 ASTM F136)
Instrument material
• Stainless steel, silicone and TA6V
Indications
This medical device is used (and only) to treat cervical disc diseases defined as refractory radiculopathy (irradiant pain) and/or myelopathy (weakness) with herniated disc and/or formation of osteophyte and/or spinal cord compression.
It has a graft chamber intended to be filled with bone substitute, wide enough to achieve a good bone graft.
ACIFBOX implants can be used in skeletally mature individuals (adults) and in adolescent.