Informations
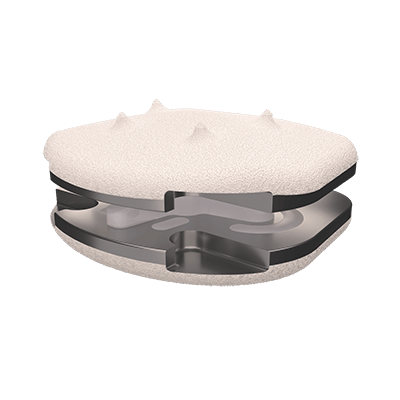
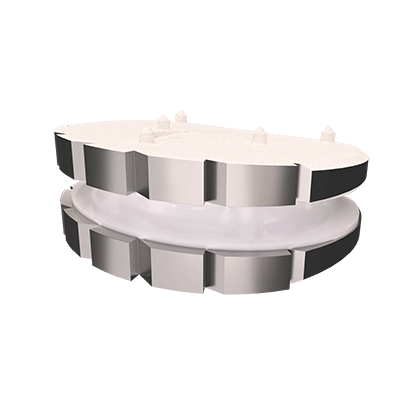
The CP-ESP® disc prosthesis is intended to replace a damaged or diseased disc between two vertebrae of the cervical spine.
The CP-ESP® disc prosthesis is designed to reproduce the natural behavior and physiological functions of a healthy disc.
Thanks to its viscoelastic PCU core, the CP-ESP® enable mimic natural motion in all six degrees of freedom (flexion-extension, lateral bending, translation, axial rotation and shock absorption or load bearing)
This flexible cushion, attached to the two metal endplates, provides the disc with its shock-absorbing function and limits the release of particles into the body and the calcification of deformable parts.
Indications
The CP-ESP® cervical disc prosthesis is designed for surgical treatment of degenerative disc disease (DDD) of the cervical spine in skeletally mature patients who have not responded after at least 6 months of conservative treatment. The assessment of the extent of disc involvement is based on the combination of data from the clinical examination and/or the symptoms and imaging.
The clinical examination and symptoms are defined by pain and/or a functional/neurological deficit in the neck or arm, with at least one of the following:
• Herniated disc
• Spondylarthrosis (defined by the presence of osteophytes)
• Radiculopathy
• Spinal cord compression.
The CP-ESP® cervical disc prosthesis is a medical device for primary treatment.