Informations
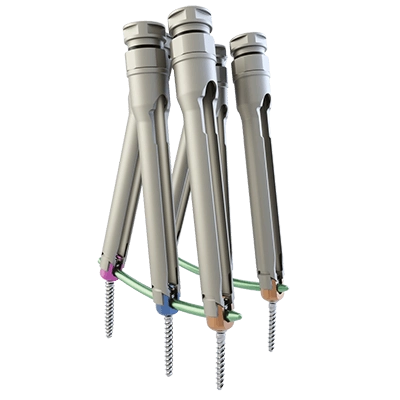
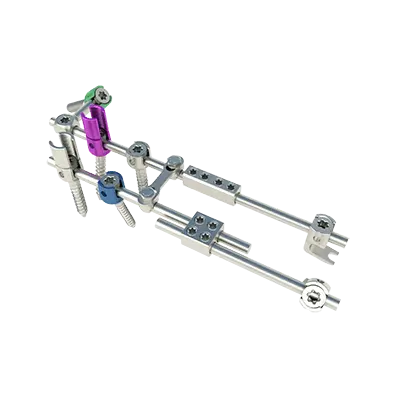
The MONT BLANC MIS implants consist of various forms and sizes of screws, locking screws and rods.
The MONT BLANC MIS implants can be combined for creating a posterior spinal implant system in a minimally invasive approach.
They are used with dedicated instruments which allow preparation of the site, insertion of implants and removal.
The MONT BLANC MIS implants are supplied for single use.
Implants material
• Titanium alloy (ISO 5832-3).
Instruments material
• Stainless steel, silicone, polyphenyl sulfone, POM, nitinol, ABS and polypropylene.
Indications
The MONT BLANC MIS System is intended for noncervical pedicle fixation and nonpedicle fixation for the following indications: degenerative disc disease (defined as back pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies); spondylolisthesis; trauma (i.e., fracture or dislocation); spinal stenosis; curvatures (i.e., scoliosis, kyphosis, and/or lordosis); tumor; pseudoarthrosis; and failed previous fusion in skeletally mature patients.
When used for posterior non-cervical pedicle screw fixation in pediatric patients, the MONT BLANC and MONT BLANC MIS Spinal Systems metallic implants are indicated as an adjunct to fusion to treat adolescent idiopathic scoliosis. The MONT BLANC and MONT BLANC MIS Spinal Systems are intended to be used with autograft and/or allograft. Pediatric pedicle screw fixation is limited to a posterior approach.