Informations
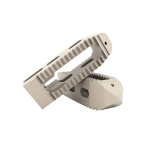
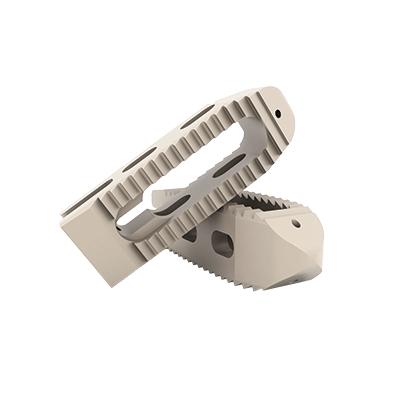
The TWIN PEAKS TLIF OBLIQUE lumbar interbody fusion system is an internal spinal fixation system intended to provide stabilization of the spine while biologic fusion occurs.
The TWIN PEAKS TLIF OBLIQUE is inserted from a posterolateral approach.
The TWIN PEAKS TLIF OBLIQUE cages are used with dedicated instruments which allow preparation of the site, insertion of implants and removal.
The TWIN PEAKS TLIF OBLIQUE cages are supplied for single use.
Implant material
• Polyetheretherketone (PEEK Optima LT1, ASTM F-2026) and Tantalum (ASTM F-560).
Instruments material
• Silicone, stainless steel, Radel® and Propylux®.
Indications
The TWIN PEAKS TLIF OBLIQUE lumbar interbody fusion system is indicated for intervertebral body fusion procedures in skeletally mature patients with degenerative disc disease (DDD) of the lumbar spine at one or two contiguous levels from L2-S1. Degenerative disc disease is defined as discogenic back pain with degeneration of the disc confirmed by history and radiographic studies.
These DDD patients may have up to Grade 1 spondylolisthesis or retrolisthesis at the involved level(s).
TWIN PEAKS TLIF OBLIQUE lumbar interbody fusion system is to be used with autogenous bone graft.
Patients should be skeletally mature and have at least six months of non-operative treatment.
The TWIN PEAKS TLIF OBLIQUE lumbar interbody fusion system must be used with supplemental fixation cleared by FDA for use in the lumbar spine.