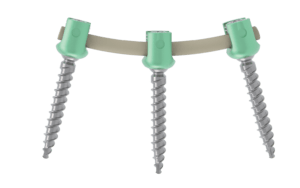
KAPHORN
KAPHORN is a side loading posterior osteosynthesis system.
It is intended for the reduction of pathologies of the thoraco-lombo-sacral spine by restoring the disc height as well as the physiological bending of the spine
Implant material: titanium alloy TA6V (ISO 5832-3 and ASTM F136).
Instrument material : stainless steel (EN 10088-2, EN 10216-5, ASTM A213), titanium (5832-3 or 5832-2), polymers
KAPHORN system can be used in skeletally mature individuals (adults) and in adolescent population for specific indications such as scoliosis.
This medical device is intended for the posterior fixation of the thoracic, lumbar and sacral spine. It is indicated for the treatment of
- deformities (any etiology)
- Trauma
- Tumors
- the degenerative spine (spondylolisthesis, degenerative disc disease, spinal fractures, spinal stenosis, non-union)
KAPHORN can also be used in revision surgery in case of failure of a previous procedure.
KAPHORN system can be used in skeletally mature individuals (adults) and in adolescent population for specific indications such as scoliosis.
This medical device is intended for the posterior fixation of the thoracic, lumbar and sacral spine. It is indicated for the treatment of deformities (any etiology), trauma, tumors, and the degenerative spine (spondylolisthesis, degenerative disc disease, spinal fractures, spinal stenosis, non-union). KAPHORN can also be used in revision surgery in case of failure of a previous procedure.
Please read the informations in the instructions for use carefully.
KAPHORN implants are class IIb healthcare products marked CE2803 under the relevant European regulations.
For proper implantation, KAPHORN must be used with a specific set of instruments and reusable Class I surgical instruments marked CE/CE0197 under the appropriate European regulations. The use of these instruments is described in the surgical technique.
June 2024
Manufacturer: DISTIMP SAS 7, allée du Moulin Berger 69130 Ecully, France